Prev Page--Geology || Next Page--Geologic Formations
Subsurface Water
All water present below the surface of the earth is called subsurface water to distinguish it from surface water in ponds, lakes, and streams.
Suspended Water
Above a certain level the voids or pore spaces in the earth are filled partly with air or other gases and partly with water. This zone is called the zone of aeration and the water in this zone is called suspended water (Fig. 5). This water may be percolating downward or it may be held in suspension by molecular attraction. Although this water is not available to springs and wells, it is of great importance because the portion of it near the surface is the chief source of moisture for plants.
Figure 5--Diagram showing divisions of subsurface water. (From O.E. Meinzer)
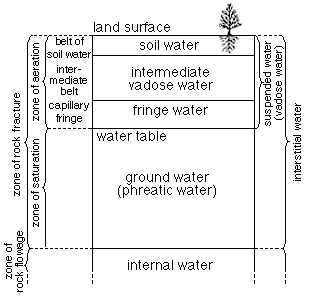
Ground Water
All voids below the zone of aeration are filled with water and this zone is called the zone of saturation. The upper surface of the zone of saturation is known as the water table. The walls of a pit or well may be moist at various levels above the water table, but water will not flow into a well until the zone of saturation is reached. All water below the water table is designated ground water. The zone of saturation extends downward to the greatest depth at which interconnected voids occur.
Principles of Occurence
This discussion on the principles of occurrence of ground water is based on a discussion by Meinzer (1923), to which the reader is referred for more complete information.
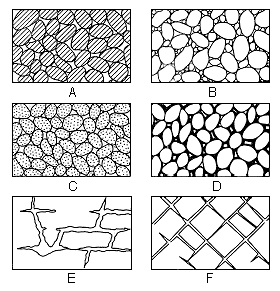
The porosity of a rock is its property of containing interstices. Porosity is expressed quantitatively as the percent of the total volume that is occupied by interstices or voids. Pore spaces fall into two general classes: (1) the open spaces between component particles (primary interstices) and (2) joints, crevices, openings along bedding planes, and solution cavities that have developed since deposition (secondary interstices). The amount of water that can be stored in a material depends upon its porosity. Several common types of open spaces or interstices, and the relation of texture to porosity are shown in Figure 6.
Figure 6--Diagram showing several types of rock interstices and the relation of rock texture to porosity. A, Well-sorted sedimentary deposit having high porosity; B, poorly sorted sedimentary deposit having low porosity; C, well-sorted sedimentary deposit consisting of pebbles that are themselves porous, so that the deposit as a whole has a very high porosity; D, well-sorted sedimentary deposit whose porosity has been diminished by the deposition of mineral matter in the interstices; E, rock rendered porous by solution; F, rock rendered porous by fracturing. (From O.E. Meinzer.)
Not all the water in the zone of saturation is available for recovery through wells. A part of the water will drain into wells by gravity, and a part will remain in the interstices of the rock formation, held by molecular attraction. The water-yielding capacity of a saturated rock is called its specific yield. The specific yield is the ratio of the volume of water yielded to the total volume of rock and is expressed as a percentage. Thus if 100 cubic feet of saturated rock yields 10 cubic feet of water by gravity the specific yield is 10 percent. If 15 cubic feet of water remained in the interstices the specific retention of the rock would be 15 percent. The sum of the specific yield and the specific retention is equal to the porosity, in this case 25 percent. A saturated rock having a specific yield of zero will yield no water. A rock formation that will yield water in sufficient quantity to be of consequence is called an aquifer.
Source
In Jackson County essentially all ground water is derived from precipitation in the form of rain or snow. Part of the moisture that falls as rain or snow is carried away by surface runoff to streams. Part of it may evaporate or be absorbed by vegetation and transpired into the atmosphere. The part that escapes discharge by these means percolates slowly downward to the water table and becomes ground water. The amount of water discharged by runoff depends upon several factors: (1) the slope of the land surface, (2) the permeability of the surficial materials, (3) amount of moisture already held in the zone of aeration, and (4) whether the surface material is frozen. The amount discharged by evaporation and transpiration depends primarily upon the temperature, humidity, and kind of vegetation.
Artesian Conditions
Ground water, under normal atmospheric pressure, will rise only as high as the water table. Where ground water is confined below an impermeable stratum and will rise above the bed in which it is contained, the water is said to be under artesian pressure. A well that flows at the land surface is a flowing artesian well.
Although no flowing wells are known in Jackson County at the present time, the water in many wells in the area is under artesian pressure.
The Water Table
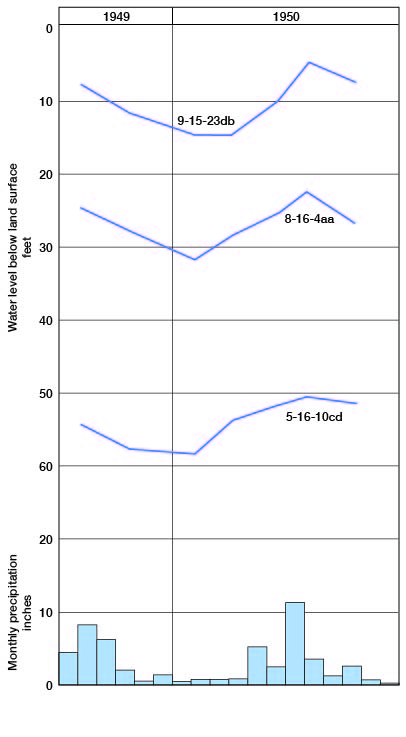
The water table is the upper boundary of the zone of saturation in ordinary permeable material. If this boundary is formed by an impermeable bed, the water table is absent. In some places the downward percolation of water within the zone of aeration may be impeded by an impermeable bed. The accumulation of water above the impermeable bed forms a local zone of saturation within the zone of aeration, known as a perched water body. The water table is not a plane surface; it differs from place to place in shape and depth below the land surface. In general the slope of the water table is similar to the slope of the land surface, except that changes in elevation are not so abrupt. In areas where the saturated material has a low permeability, the slope of the water table is much steeper than in areas of high permeability, other conditions being equal. Heavy pumping of wells will temporarily cause a local lowering of the water table, whereas recharge from a stream will cause the water table to be higher along the stream. The water table is nearer the surface during and immediately following periods of heavy rainfall (Fig. 7).
Figure 7--Hydrographs showing the fluctuation of water level in three wells in Jackson County.
As shown by the geologic cross sections (Pl. 3), the bedrock floor of the area overlain by thick glacial deposits slopes in the same general direction as the land surface. The direction of general movement of ground water in this area is eastward. South of Straight Creek the ground water moves northeast, and north of Straight Creek it moves southeast. In areas where the bedrock is exposed, or is covered with a thin mantle of unsaturated material, a water table does not exist, any ground water present being in the form of confined or artesian water. Although there is no water table, there is an imaginary surface, the piezometric surface, which coincides with the level to which water will rise in artesian wells and which, like the water table, shows the direction of movement of ground water and the effects of recharge and discharge.
Ground-water Recharge
Recharge is the addition of water to the zone of saturation. The sources of recharge in Jackson County are precipitation, streams, and subsurface flow.
Recharge by precipitation--Most of the ground water available to wells and springs in Jackson County falls on the area as rain or snow. The zone of aeration must absorb more water than can be held up by capillary forces before the zone of saturation receives recharge from precipitation; thus, if the soil moisture is nearly depleted, a moderate amount of precipitation may not recharge the groundwater reservoir. Conditions for ground-water recharge by precipitation are unfavorable over large areas of Jackson County where glacial till is the predominant surficial material. Because of the low permeability of the till much of the water of a heavy rainfall is lost by surface runoff. Thick deposits of sand and gravel at or near the surface are often found incorporated with glacial drift. Such deposits offer ideal conditions for recharge but are not nearly as extensive as the till. Recharge by precipitation in bedrock areas takes place at the outcrop of permeable beds of dipping limestone or sandstone.
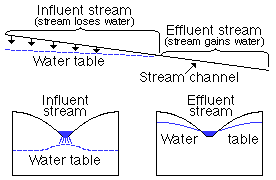
Recharge by streams--The recharge of ground water by streams is not important in Jackson County. An intermittent or ephemeral stream is one that flows only during periods of heavy rainfall. The channel of such a stream is not cut down to the water table, and when the stream is flowing some water seeps into the stream bed and percolates downward to the water table. A stream that loses water to the zone of saturation is called an influent stream, and a stream that gains water from the zone of saturation is called an effluent stream. Influent and effluent streams are illustrated by the diagrammatic sections in Figure 8.
Figure 8--Diagrammatic sections showing influent and effluent streams.
Recharge by subsurface flow--The movement of ground water in northern Jackson County is to the east; hence, some ground water moves into Jackson County from the area to the west by subsurface flow. Water confined in a permeable bed by an overlying impermeable bed moves generally in the direction of regional dip; hence, some water is derived from areas outside Jackson County in this manner.
Discharge of Ground Water
Ground-water discharge is the removal of water from the zone of saturation, and may take place by transpiration and evaporation, by discharge from springs and seeps, by subsurface flow into an adjoining, area, and by pumping from wells.
Discharge by transpiration and evaporation--The roots of plants may extend down to the water table or capillary fringe and discharge the water into the atmosphere by transpiration. In areas where the water table is far below the surface, only the deep-rooted plants known as phreatophytes are able to withdraw water from the zone of saturation. However, where the water table is near the surface, as in the valleys of Jackson County, the ordinary grasses and field crops can withdraw ground water by transpiration.
Water is lost directly by evaporation in places where the water table is at the surface, such as streams, ponds, and swampy areas.
Discharge by springs and seeps--A stream whose channel has cut below the water table will receive ground water from springs and seeps and is said to be a gaining or effluent stream. The perennial streams of Jackson County are of the effluent type, except possibly during long periods of drought when the water table is lower. Seeps may be noted along the banks of many creeks and ditches in Jackson County, generally where the downward percolation of water has been interrupted by an impermeable formation. Several of the larger springs in Jackson County are listed in Table 10.
Discharge by subsurface flow--The discharge of ground water from Jackson County by subsurface flow is into the area to the east and is probably about equal to the amount entering the county from the west.
Discharge by wells--Practically all the domestic and stock supplies of water in Jackson County are derived from wells. Although wells are the most obvious method of discharge, the amount of water withdrawn by wells is relatively small.
Recovery
Principles of recovery--When water is removed from a well the water table or piezometric surface is lowered in an area encircling the well, resulting in an inverted cone-shaped depression. This depressed area is known as the cone of depression. The amount of lowering of the water table at the well is called the drawdown. As the pumping rate of the well is increased, the drawdown becomes greater. When a well is first pumped the water level falls very rapidly, but as pumping is continued the drawdown increases at a diminishing rate. When pumping is stopped the recovery is rapid at first, but gradually tapers off and may continue for many hours or days after pumping is stopped.
The specific capacity of a well is the rate of yield per unit of drawdown and is generally expressed in gallons a minute per foot of drawdown. In testing the specific capacity of a well, pumping is continued until the water level remains approximately stationary, or for some arbitrary period such as 24 hours.
Construction of wells--In much of the area of Jackson County where shallow wells obtain water from consolidated rocks, dug wells are the most common type. This type of well is simply a pit dug into the water-bearing rocks and walled up with rock, brick, or concrete. The advantage of this type of well is the large infiltration area and storage reservoir provided by the large diameter. Dug wells are more subject to contamination and failure during dry weather than deeper drilled wells.
In some of the valleys containing alluvium, a few driven wells supply stock and domestic needs. Driven wells can be used only where the water table is near the surface, and where the material is soft enough to permit a pipe to be driven to the water table. A driven well consists of a length of 1 1/4 or 1 1/2 inch pipe having a drive-point screen on the lower end. They are usually pumped by a pitcher pump. The aquifer must be quite permeable for a satisfactory driven well because the intake area of the drive point is small.
Most of the wells in the thick drift area of the county, as well as many of the deeper wells in other parts of the county, are of the drilled type. Wells may be drilled either by the percussion method or by hydraulic-rotary machines. The drilled wells for stock and domestic use are usually 6 inches in diameter and are cased with galvanized-steel or wrought-iron casing. Wells obtaining water from unconsolidated material are cased to the bottom. The portions of the casing that are in the water-bearing beds are perforated, or a specially designed screen is used to allow intake of water. Many drilled wells obtaining water from consolidated beds that will not cave are left uncased in the lower part. Some municipal and industrial wells in unconsolidated material are gravel-packed. In this type of construction a large-diameter hole is first made and cased. A smaller casing containing sections of well screen spaced to correspond with the water-bearing beds is then centered in the hole and the annular space between the large casing and the smaller casing is filled with carefully selected gravel. The larger casing is then withdrawn to permit the water to flow into the well. This type of construction increases the effective diameter of the well and helps to prevent fine sand from entering the well.
Several of the wells visited in Jackson County were bored by means of a well auger and are fitted with bell-top clay-tile casing about 14 inches in diameter. They are generally shallow and are more subject to contamination than drilled wells.
Utilization of Water
Domestic and stock supplies--Practically all the domestic and stock supplies of water in the county are derived from wells or springs. In areas where relatively large supplies of water of good quality are not available, many farms have shallow wells near the house for domestic use and a deeper well some distance from the house for stock supplies. Many of the stock farms have ponds for stock water formed by damming natural drainageways.
Public supplies--Holton is the only city in Jackson County having a public water-supply system. Until 1950 the water supply of Holton was derived from four wells and nine springs. Two of the wells are east of the city in the alluvium of Elk Creek. One of these wells (6-15-35dd) is 48 feet deep, is cased with 6-inch iron casing, and yields about 20 gallons per minute. The other well (6-15-36dd) is 38 feet deep, is cased with 6-inch iron casing, and yields 48 gallons per minute.
The springs and other wells are about a mile north of well 6-15-36dd and derive their water from glacial sand and gravel. They yield 12 to 55 gallons per minute each. Vertical-turbine pumps powered by small electric motors pump the water from the springs into a central sump. The system has storage facilities totaling 750,000 gallons. The average daily consumption is about 200,000 gallons, of which 35,000 gallons is used by the Chicago, Rock Island, and Pacific Railroad. Since 1950 the city has depended on impounded surface water for its water supply.
Irrigation and industrial supplies--No irrigation is practiced in Jackson County, and no industries have their own water supply.
Quality of Water
The chemical character of the ground water in Jackson County is indicated by the analyses in Table 4 and Figure 9. The analyses were made by H. A. Stoltenberg in the Water and Sewage Laboratory of the Kansas State Board of Health. Twenty-four samples of water were collected from wells distributed fairly uniformly over the area, deriving water from the principal aquifers within the county.
Table 4--Analyses of water from typical wells in Jackson County. Analyzed by H. A. Stoltenberg. Dissolved constituents given in parts per million. One part per million is equivalent to one pound of substance per million pounds of water or 8.33 pounds per million gallons of water. An equivalent per million is a unit chemical equivalent weight of solute per million unit weights of solution. Concentration in equivalents per million is calculated by dividing the concentration in parts per million by the chemical combining weight of the substance or ion.
| Well number |
Depth (feet) |
Geologic source |
Date of collection |
Temperature °F |
Dissolved solids |
Silica (SiO2) |
Iron (Fe) |
Calcium (Ca) |
Magnesium (Mg) |
Sodium and Potassium (Na + K) |
Bicarbonate (HCO3) |
Sulfate (SO4) |
Chloride (Cl) |
Fluoride (Fl) |
Nitrate (NO3) |
Hardness as CACO3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Carbonate | Noncarbonate | ||||||||||||||||
| 5-15-16bb | 219 | Glacial sand and gravel | 4/2/1951 | 54 | 1,660 | 31 | 2.2 | 170 | 67 | 307 | 400 | 567 | 316 | 0.5 | 2.4 | 700 | 328 | 372 |
| 5-15-31cd | 102 | Glacial sand and gravel | 3/27/1951 | 56 | 1,280 | 26 | 0.24 | 203 | 58 | 137 | 405 | 380 | 157 | 0.3 | 115 | 745 | 332 | 413 |
| 5-16-15cd | 20 | Dry-Friedrich shale | 4/2/1951 | 55 | 536 | 10 | 0.18 | 109 | 25 | 29 | 271 | 31 | 39 | 0.2 | 159 | 375 | 222 | 153 |
| 5-16-20dd | 44 | Glacial sand and gravel | 4/2/1951 | 54 | 486 | 22 | 0.2 | 75 | 24 | 69 | 420 | 16 | 23 | 0.7 | 49 | 286 | 286 | 0 |
| 6-12-1aa | 20 | Beattie limestone | 3/27/1951 | 392 | 6.2 | 0.21 | 88 | 11 | 32 | 256 | 42 | 16 | 0.3 | 71 | 264 | 210 | 54 | |
| 6-13-8cc | 38 | Glacial sand and gravel | 4/2/1951 | 54 | 791 | 24 | 0.71 | 117 | 42 | 95 | 464 | 15 | 93 | 0.3 | 177 | 464 | 380 | 84 |
| 6-13-18aa | 85 | Beattie limestone | 4/2/1951 | 55 | 678 | 15 | 0.42 | 126 | 52 | 29 | 390 | 210 | 15 | 0.4 | 39 | 528 | 320 | 208 |
| 6-14-27bb | 47 | Foraker limestone | 3/28/1951 | 54 | 649 | 14 | 0.48 | 113 | 31 | 65 | 364 | 154 | 27 | 0.4 | 66 | 410 | 298 | 112 |
| 6-15-8ad | 51 | Glacial sand and gravel | 4/2/1951 | 55 | 331 | 15 | 0.97 | 82 | 13 | 12 | 239 | 25 | 13 | 0.3 | 53 | 258 | 196 | 62 |
| 6-15-22cd | 96 | Glacial sand and gravel | 3/28/1951 | 56 | 535 | 12 | 0.13 | 93 | 33 | 54 | 376 | 123 | 32 | 0.4 | 2.5 | 368 | 308 | 60 |
| 6-16-36cb | 40 | Burlingame and Wakarusa limestones | 4/3/1951 | 56 | 911 | 9.2 | 0.42 | 118 | 51 | 71 | 183 | 27 | 67 | 0.1 | 478 | 504 | 150 | 354 |
| 7-13-10bb | 75 | Foraker limestone | 3/27/1951 | 549 | 12 | 0.29 | 118 | 33 | 28 | 381 | 133 | 14 | 0.3 | 23 | 430 | 312 | 118 | |
| 7-13-23aa | 57 | Red Eagle limestone | 4/2/1951 | 56 | 383 | 8.2 | 0.86 | 97 | 23 | 11 | 354 | 35 | 10 | 0.4 | 24 | 336 | 290 | 46 |
| 7-13-33ad | 46 | Grenola limestone | 3/27/1951 | 56 | 467 | 9.8 | 0.25 | 87 | 42 | 23 | 420 | 33 | 12 | 0.3 | 53 | 390 | 344 | 46 |
| 7-14-11aa | 69 | Glacial sand and gravel | 3/28/1951 | 57 | 319 | 18 | 0.43 | 74 | 20 | 12 | 290 | 22 | 12 | 0.3 | 18 | 266 | 238 | 28 |
| 7-14-27cc | 98 | Red Eagle and Grenola limestones | 3/28/1951 | 55 | 422 | 11 | 0.18 | 84 | 23 | 35 | 354 | 13 | 16 | 0.2 | 66 | 304 | 290 | 14 |
| 7-14-29dd | 67 | Red Eagle limestone | 3/28/1951 | 55 | 431 | 9 | 0.23 | 98 | 27 | 20 | 368 | 52 | 12 | 0.3 | 32 | 356 | 302 | 54 |
| 7-15-11cc | 70 | Dry-Friedrich shale. | 3/28/1951 | 52 | 329 | 15 | 0.7 | 80 | 12 | 21 | 290 | 16 | 9 | 0.2 | 33 | 249 | 238 | 11 |
| 7-15-29bb | 51 | Glacial sand and gravel | 4/2/1951 | 55 | 640 | 24 | 0.91 | 99 | 41 | 63 | 386 | 192 | 18 | 0.7 | 12 | 416 | 316 | 100 |
| 8-14-10ad | 60 | West Branch and Hamlin shales | 4/3/1951 | 55 | 482 | 27 | 0.24 | 82 | 30 | 50 | 420 | 49 | 18 | 0.4 | 19 | 328 | 328 | 0 |
| 8-16-9dd | 50 | Glacial sand and gravel | 4/3/1951 | 56 | 758 | 21 | 0.25 | 119 | 47 | 84 | 393 | 155 | 128 | 0.6 | 9.7 | 490 | 322 | 168 |
| 9-13-27da | 30 | French Creek shale | 3/27/1951 | 397 | 6.6 | 0.17 | 80 | 35 | 19 | 378 | 41 | 12 | 0.4 | 17 | 344 | 310 | 34 | |
| 9-13-28ad | 31 | Terrace Deposits | 3/27/1951 | 57 | 1,090 | 15 | 0.25 | 162 | 34 | 185 | 446 | 102 | 290 | 0.1 | 80 | 544 | 366 | 178 |
| 9-16-30dc | 115 | Cedar Vale and Silver Lake shales | 4/2/1951 | 55 | 520 | 8.2 | 0.64 | 18 | 9.6 | 174 | 499 | 77 | 9 | 0.4 | 2.8 | 84 | 84 | 0 |
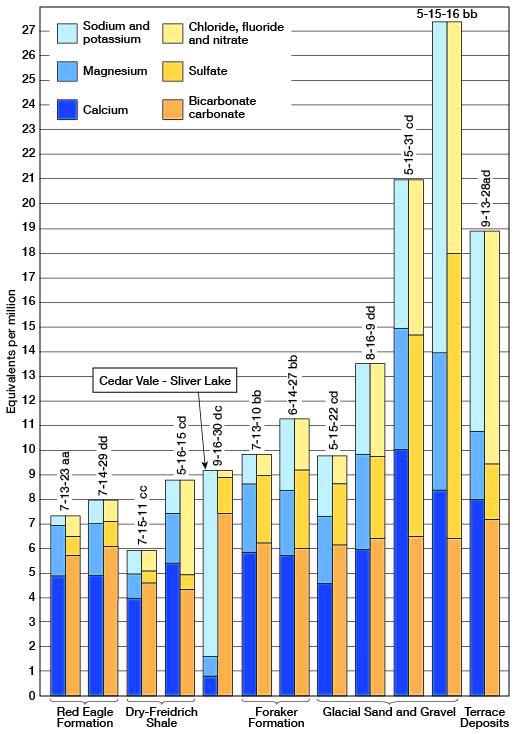
Figure 9--Analyses of water from some part of the principal water-bearing formations in Jackson County.
Chemical Constituents in Relation to Use
The following discussion of the chemical constituents of ground water in relation to use has been adapted in part from publications of the U. S. Geological Survey and the State Geological Survey of Kansas.
Dissolved solids--The residue left after a natural water has evaporated consists of rock materials and may include some organic material and some water of crystallization. Waters containing less than 500 parts per million of dissolved solids are generally satisfactory for domestic use, except for the difficulties resulting from their hardness and, in some areas, excessive iron content and corrosiveness. Waters having more than 1,000 parts per million of dissolved solids are generally not satisfactory for domestic use, for they are likely to contain enough of certain constituents to produce a noticeable taste or to make the water unsuitable in some other respects.
The dissolved solids in samples of water from Jackson County ranged from 319 to 1,660 parts per million. A little less than half the samples contained less than 500 parts per million and about two-fifths contained between 500 and 1,000 parts per million (Table 5). Only three of the samples contained more than 1,000 parts per million. The samples having the greatest amount of dissolved solids were from deep wells deriving water from glacial sand and gravel.
Table 5--Dissolved solids in samples of water from wells in Jackson County.
| Dissolved solids, parts per million |
Number of samples |
|---|---|
| less than 300 | 0 |
| 301-400 | 6 |
| 401-500 | 5 |
| 501-600 | 4 |
| 601-700 | 3 |
| 701-800 | 2 |
| 801-900 | 0 |
| 901-1,000 | 1 |
| more than 1,000 | 3 |
| Total | 24 |
Hardness--The hardness of water, which is the property that generally receives the most attention, is recognized most commonly by its effects when soap is used with the water. Calcium and magnesium cause almost all the hardness of ordinary water. These constituents are also the active agents in the formation of the greater part of the scale formed in steam boilers and other vessels used to heat or evaporate water.
In addition to the total hardness, the table of analyses shows the carbonate hardness and the noncarbonate hardness. The carbonate hardness is due to the presence of calcium and magnesium bicarbonates and can be almost completely removed by boiling. In some reports this type of hardness is called temporary hardness. The permanent or noncarbonate hardness is due to the presence of sulfates or chlorides of calcium and magnesium and cannot be removed by boiling. With reference to use with soap, the carbonate hardness and noncarbonate hardness do not differ. In general, the noncarbonate hardness forms harder scale in steam boilers.
Water having a hardness of less than about 50 parts per million is generally rated as soft, and treatment for the removal of hardness is not necessary under ordinary circumstances. Hardness between 50 and 150 parts per million does not seriously interfere with the use of water for most purposes, but the hardness does slightly increase the amount of soap used and removal by a softening process is profitable for laundries or other industries using large quantities of soap. Water having a hardness in the upper part of this range will cause considerable scale on steam boilers. Hardness above 150 parts per million is very noticeable, and if the hardness is more than about 200 parts per million water for household use is commonly softened. Where municipal water supplies are softened an attempt is made generally to reduce the hardness to about 80 to 90 parts per million. The additional improvement from further softening of a public supply generally is not deemed worth the increase in cost.
Water samples collected in Jackson County ranged in total hardness from 84 to 745 parts per million. Only 1 sample contained less than 200 parts per million (Table 6), 21 samples contained 200 to 600 parts per million, and 2 samples contained more than 600 parts per million total hardness.
Table 6--Hardness of samples of water from wells in Jackson County.
| Hardness, parts per million |
Number of samples |
|---|---|
| less than 100 | 1 |
| 101-200 | 0 |
| 201-300 | 5 |
| 301-400 | 8 |
| 401-500 | 5 |
| 501-600 | 3 |
| 601-700 | 1 |
| 701-800 | 1 |
| Total | 24 |
Iron--Next to hardness, iron is the constituent of natural water that generally receives the most attention. The quantity of iron in ground water may differ greatly from place to place, even though the water may be derived from the same formation. If a water contains more than a few tenths of a part per million of iron, the excess may settle out as a reddish precipitate. Iron, present in sufficient quantity to give a disagreeable taste and to stain cooking utensils and plumbing, may be removed from most water by simple aeration and filtration, but some water requires the addition of lime or some other substance. "Zeolite-type" filters also can be used.
The iron content of the water samples from wells in Jackson County ranged from 0.17 to 13 parts per million. Twenty of the samples contained less than 1 part per million of iron; two samples contained more than 2 parts per million (Table 7).
Table 7--Iron content of samples of water from wells in Jackson County.
| Iron, parts per million |
Number of samples |
|---|---|
| 0.0-0.10 | 0 |
| 0.11-0.30 | 12 |
| 0.31-0.50 | 4 |
| 0.51-0.70 | 2 |
| 0.71-1.00 | 4 |
| 1.1-2.0 | 0 |
| 2.1-3.0 | 1 |
| more than 3.0 | 1 |
| Total | 24 |
Fluoride--The fluoride content of waters likely to be used by children should be known because fluoride in water is associated with the dental defect known as mottled enamel, which may appear on the teeth of children who drink water containing excessive amounts of fluoride during the period of formation of the permanent teeth. Waters containing more than about 1.5 parts per million of fluoride are likely to produce mottled enamel. If the water contains as much as 4 parts per million of fluoride, 90 percent of the children are likely to have mottled enamel, and 35 percent or more of the cases will be classified as moderate or worse (Dean, 1936). However, contents of fluoride up to I part per million are believed by many health authorities to be beneficial in inhibiting tooth decay.
None of the samples of water from wells in Jackson County contained as much as 1 part per million of fluoride.
Nitrate--The use of water containing an excessive amount of nitrate in the preparation of a baby's formula can cause cyanosis or oxygen starvation ("blue babies"). Some authorities advocate that water containing over 45 parts per million of nitrate (as NO3) should not be used in formula preparation (Metzler and Stoltenberg, 1950). Water containing 90 parts per million of nitrate is generally considered very dangerous to infants, and water containing 150 parts per million may cause severe cyanosis. Cyanosis is not produced in adults and older children by the concentrations of nitrate found in drinking water. Boiling water high in nitrate content does not render it safe for use by infants; therefore, only water that is known to be low in nitrate content should be used for this purpose.
The nitrate content of the water from a well may be somewhat seasonal, being highest in the winter and lowest in the summer (Metzler and Stoltenberg, 1950, p. 201). In general, water from wells that are susceptible to surface contamination is likely to be high in nitrate concentration.
The nitrate content of the water from wells sampled in Jackson County ranged from 2.4 to 478 parts per million. Thirteen of the samples contained less than 40 parts per million of nitrate, 7 contained 40 to 80 parts per million, and 4 contained more than 80 parts per million of nitrate (Table 8). In general, water from the deeper drilled wells deriving water from glacial sand and gravel had the lowest nitrate content.
Table 8--Nitrate content of samples of water from wells in Jackson County.
| Nitrate, parts per million |
Number of samples |
|---|---|
| 0-10.0 | 4 |
| 10.1-20.0 | 4 |
| 20.1-30.0 | 2 |
| 30.1-40.0 | 3 |
| 40.1-60.0 | 3 |
| 60.1-80.0 | 4 |
| 80.1-100.0 | 0 |
| 100.1-200 | 3 |
| More than 200 | 1 |
| Total | 24 |
Table 9--Sulfate content of samples of water from wells in Jackson County
| Sulfate, parts per million |
Number of samples |
|---|---|
| 0-25 | 6 |
| 25-50 | 7 |
| 50-100 | 2 |
| 100-200 | 6 |
| 200-400 | 2 |
| More than 400 | 1 |
| Total | 24 |
Prev Page--Geology || Next Page--Geologic Formations
Kansas Geological Survey, Geology
Placed on web Aug. 9, 2007; originally published June 1953.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/General/Geology/Jackson/05_gw.html