
A majority of the elements are found in a variety of isotopic forms. Many of these isotopes are unstable and decay to a more stable form, while emitting radiation of several types. Gamma rays have significantly high penetrations and can be measured by simple devices such as Geiger counters or scintillation detectors on logging tools. Of the many radioactive isotopes which are known, only three types occur in any appreciable abundance in nature:
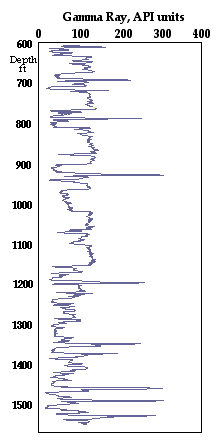
The measurement scale of the gamma-ray log is in API (American Petroleum Institute) units, accepted as the international reference standard that allows consistent comparisons to be made between a wide variety of gamma-ray counting devices. The API standard is set by the primary calibration test pit at the University of Houston where a radioactive cement calibrator is assigned a value of 200 API units and conceived originally so that a typical Midcontinent shale would register at about 100 API units.
Analyses of the North American Shale Composite (NASC) reference standard reported values of Th 12.3 ppm, U 2.66 ppm., K 3.2%, which converts to an equivalent gamma-ray log reading of 121.7 API units. Although higher than the vague assertion that a typical Midcontinent shale should read about 100 API units, the hypothetical log value of the NASC standard is a good match with the gray shales of the Pennsylvanian succession shown in the figure at right. The black shales, however, are prominent as thin anomalously radioactive zones. Their markedly different character is produced by a high U content that supplements radioactive sources in gray shales of 40K contained in illite and other K-bearing minerals, and Th contained in monazite in the silt and clay fraction and adsorbed at clay-mineral surfaces.
In the majority of stratigraphic and petroleum geological applications, the gamma ray log is used as a "shale log", both to differentiate shales and "clean" formations and to evaluate shale proportions in shaly formations. Typical sandstones, limestones and dolomites have relatively low concentrations of radioactive isotopes as contrasted with shales. Most carbonates show very low levels of radioactivity unless they contain disseminated shale or have been mineralized by uranium-bearing solutions. Simple orthoquartzites show similarly low values, although relatively high readings may be introduced by significant amounts of shale, felspar, mica or heavy minerals such as zircon.