Prev Page--Mineral resources || Next Page--Well Records
Ground-water Resources
Recharge of Ground Water
The principal source of recharge to aquifers in Allen County is precipitation on the county. Some ground water moves into and out of the county from adjacent areas. Some recharge is contributed by streams.
Precipitation
Only a small part of the precipitation on Allen County reaches the ground-water reservoir. A large percentage of the precipitation is discharged as surface runoff or returns to the atmosphere from the soil by evaporation and transpiration. The rate of precipitation, type of soil, character of underlying rocks, type and amount of vegetation, and topography all affect the rate and quantity of recharge.
Probably the most favorable conditions for recharge in Allen County occur in the alluvium and Pleistocene terrace deposits in the Neosho River valley.
A relatively rapid rise in water level follows moderate to heavy rainfall in areas directly underlain by the Iola Limestone and in areas where the Iola is an aquifer. This is probably due to recharge through an extensive joint system in the limestone. Other areas in the county either are directly underlain by relatively impermeable rocks or do not have a large enough outcrop area to be favorable for recharge.
Some recharge may occur through the black fissile shales of the Dennis and Swope Limestones in the extreme northeast and southeast corners of the county.
Adjacent Areas
Subsurface movement of water from areas outside of Allen County is probably a relatively unimportant source of recharge to the groundwater reservoir. The black shales of the Dennis and Swope Limestones mentioned above direct some water into Allen County from Neosho and Bourbon counties.Streams
Some water may reach the ground-water reservoir from streams during periods of high stage. However, when the stage of the stream drops below the level of the water table in the aquifer, the direction of ground-water flow is reversed, and the water is discharged from the aquifer into the stream. Flow in the smaller streams a week or more after rains probably is maintained by discharge from the ground-water reservoir.
The drainage area of the Neosho River above Iola is 3,818 square miles. If an average rainfall of 36 inches is assumed, then about 7.33 million acre-feet of precipitation is received annually for this area. The mean annual direct runoff for this area is about 1.02 million acre-feet (Busby and Armentrout, 1965), or 14 percent of the annual precipitation.
Discharge of Ground Water
In Allen County, ground water is discharged by seepage into streams, by evaporation and transpiration, by springs and wells, and by subsurface movement to adjacent areas. Climate and the stage of the water table control the rate of natural discharge. More water is discharged in some parts of the county than in others owing to local differences in geology and topography. Only a small part of the ground-water discharge in Allen County is pumped from wells. In this report, mean annual base flow in the Neosho River is assumed to be at least 0.23 million acre-feet and is about equal to mean annual recharge to the alluvial deposits. In other words, base flow is considered to result from ground-water discharge into the streams.
Evaporation and Transpiration
More ground water is discharged by evaporation and transpiration than by all other means combined. Where the water table is near the land surface, direct evaporation of ground water occurs. Ground water is also transpired by many plants. The zone of saturation or the capillary fringe above it is penetrated by the roots of many plants in the stream valleys. In upland areas, the water table is relatively deep and discontinuous, and the ground-water reservoir is tapped by only a few plants. A fairly representative figure for loss by evaporation and transpiration (6.08 million acre-feet per year or about 29.8 inches) can be obtained by subtracting from total precipitation the combined amounts for mean base flow and mean direct runoff.
Subsurface Movement
Subsurface movement of ground water into or from adjacent areas is relatively unimportant. Some water leaves the county through the alluvium and terrace deposits in the Neosho River valley and moves into Neosho County. A small amount of water leaves through consolidated rock aquifers across the western and northern borders of the county owing to the effect of the regional dip of the sediments.
Seeps and Springs
Ground water is discharged by seeps and springs along valley walls and in upland areas. Water that remains after evaporation and transpiration by plants during the growing season flows in streams and leaves the county as surface runoff.
Wells
Three types of wells are used to obtain water supplies in Allen County: dug wells, driven wells, and drilled wells. The type of well constructed depends mainly on the use for which the well is intended, the geologic materials to be penetrated, the depth to water, and the depth to which the well must penetrate.
Dug wells are large-diameter wells that are excavated with either hand tools or power equipment. These wells are usually cased with rock, but tile and Concrete casings also are used. In wells of this type, the aquifer is usually penetrated only a few feet below the water table. This type of well is generally used in upland areas that are underlain by stratified deposits of Pennsylvanian age. Large-diameter dug wells are often desirable as they provide a greater storage space within the well, which compensates to some extent for a slow rate of water movement into a well constructed in deposits of low permeability. In addition, the larger storage space within the well is useful during periods of drought. The large diameter of this type of well permits a greater surface area of the aquifer to be exposed; hence, more water is available than in drilled wells in the same aquifer.
Driven wells are small-diameter wells consisting of 1 1/4- to 2-inch pipe having a screen or sandpoint attached to the bottom. The use of this type of well is limited to areas underlain by unconsolidated deposits and a relatively shallow water table. The pipe with a screen attached to the lower end is driven into the aquifer so that the screen is below the water table. The permeable gravel generally is in the lower part of the valley fill and these wells must be driven to bedrock.
Drilled wells inventoried in Allen County (table 5) range in diameter from about 4 to 12 inches and have been drilled with either percussion (cable tool) or rotary drilling machines. Wells drilled in unconsolidated deposits must be cased for their full depth and screened in the saturated zones. Wells drilled into Pennsylvanian bedrock may be uncased, except in the interval of weathered surface rock. The surface casing prevents rock in the weathered zone from falling into the well and seals out water from the surface and the weathered zone. Most drilled domestic and stock wells in the county range in diameter from 4 to 10 inches; yields of these wells range from about 1 to 5 gpm (gallons per minute).
Availability of Ground Water
Consolidated Rock Aquifers
Limestone and Shale Aquifers
Limestone and shale units at or near the land surface are widespread throughout Allen County. The unweathered limestones and shales generally are relatively impermeable and do not yield enough water to a well to provide an adequate domestic water supply. However, weathering processes at or near the land surface enlarge the open spaces within the rocks, so that they may yield 1 to 5 gpm of ground water to shallow wells. Yields of 5 to 20 gpm have been reported from wells in the limestone and shale aquifers.
Movement of water through these aquifers is slow and complex. Joint systems and fractures in the limestones at or near the land surface (fig. 9) provide channels through which recharge and movement of ground water occur (Lattman and Parizek, 1964). Figure 10 is a map showing the strike of joint patterns in outcropping limestones in Allen County. Water is assumed to move downward along these fractures and joints until it reaches the contact with the shale at the base of the limestone or a point where the fracture closes and restricts further downward movement. Unweathered shale, being relatively impermeable, acts as an aquiclude, and the water moves laterally along the shale and limestone contact. The permeability of the weathered limestones and shales differs greatly from area to area. Recharge to and discharge from limestone and shale aquifers are greatly influenced by such factors as type and thickness of soil, vegetation, slope, topographic position, as well as thickness and extent of the weathered zone.
Figure 9--Joint patterns developed in the Iola Limestone in the NW sec. 22, T. 26 S., R. 18 E.

Figure 10--Strike of Joint patterns developed in outcropping limestones.
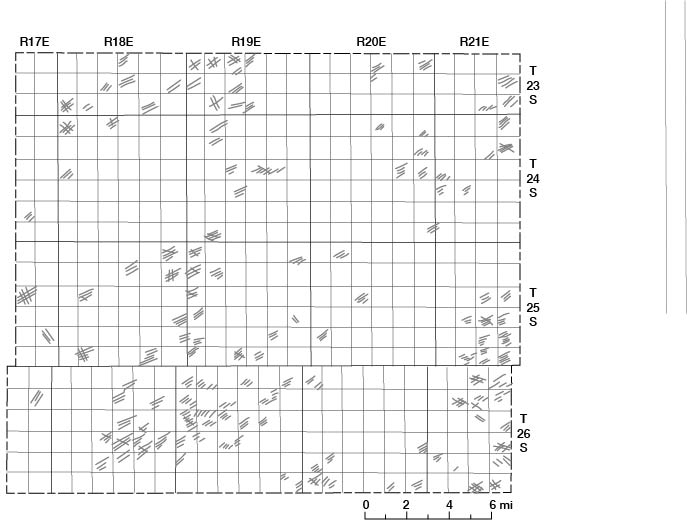
All the limestone and shale units that crop out in Allen County probably locally yield water in variable amounts to wells. Differences in permeability, degree of weathering, distance from points of recharge, well diameter, and structural attitude of the rocks govern the amount of water, if any, that can be obtained from wells.
Black fissile carbonaceous shale occurs in the Swope and Dennis Limestones, and locally in the Cherryvale Shale. These black shales yield some water to wells and locally are the principal aquifers for small domestic supplies.
The mineral or chemical quality of water from the weathered limestone and shale aquifers is generally satisfactory for domestic use, except for excessive hardness and iron content.
Sandstone Aquifers
Several of the shale units in Allen County contain intraformational sandstones that locally yield 1 to 2 gpm of ground water to domestic wells. The sandstones are similar lithologically and hydrologically, and are very fine to fine-grained micaceous quartzose sandstone with angular to subangular phenoclasts. The permeability of these sandstones is low, commonly in the magnitude of 1 to 50 gpd (gallons per day) per square foot, and most of the groundwater movement is probably along bedding planes.
Unconsolidated Rock Aquifers
Neosho River valley--Wells yielding 10 to 100 gpm can be obtained from most of the area mapped on plate 1 as Recent and Wisconsinan alluvium and Illinoisan terrace deposits in the Neosho River valley. Logs of test holes indicate that these deposits have a maximum thickness of about 35 feet, but the deposits are commonly 25 to 30 feet thick. The saturated thickness ranges from 0 to about 20 feet.
An 8-inch test hole (24-18E-28cdd) was drilled during this investigation and developed as a well. The initial saturated thickness at the well was 8.4 feet, which included 2.5 feet of silty gravel overlain by 5.9 feet of clayey silt. The well was screened using slotted pipe from a depth of 22 to 24.5 feet and was test-pumped at a rate of 15 gpm for 13 hours. The test indicated that the aquifer was artesian in the early part of the test, but after about 5 hours, water-table conditions existed in the vicinity of the pumping well. Hydraulic properties of the aquifer determined from this test are based on water-table conditions.
The results of this aquifer test indicate that the well has a specific capacity of about 26 gpm per foot of drawdown after pumping at a rate of 15 gpm for 13 hours. The aquifer has a coefficient of permeability (P) of about 5,200 gpd per square foot and a coefficient of transmissibility (T) of about 44,000 gpd per foot. Because of the thinness of the saturated material and the fact that only the lower few feet of the deposits have characteristics that give such high values for (T) and (P), longer periods of pumping would result in a decrease in saturated thickness and would appreciably decrease the average values determined for T (15,000 to 25,000 gpd per foot) and the specific capacity (7.5 to 12.5 gpm per foot of drawdown).
Test hole log 25-18E-30baa indicates that parts of the aquifer have greater thicknesses of gravel and greater saturated thicknesses than was penetrated by the pumped well. Other conditions being equal, wells yielding 50 to 100 gpm probably could be obtained in some parts of the aquifer.
During years of high water-level stages, the potential yield of wells in the aquifer would be larger than normal; conversely, during periods of low water-level stages, the potential yield of wells in the aquifer would be smaller.
In the development of industrial and municipal water supplies in this aquifer, the normal fluctuation of the water table and the additional drawdown or decline caused by pumping are important factors to be considered in determining the dependable well yield that can be expected. Drawdown of the water table by increased pumping would provide additional storage space for recharge. In parts of the valley, natural discharge to the streams would be curtailed by a low water table, thereby conserving water that would otherwise be lost.
Southwest of Iola a hydraulic connection exists between the Illinoisan terrace deposits and the alluvium (fig. 7, E-E'). Ground water moves from the terrace deposits into the alluvium, which results in much higher water levels on the west side of the river than on the east.
Movement of ground water through the unconsolidated deposits is slow, as the hydraulic gradient is only about 10 to 20 feet per mile toward the river and 2 feet per mile in a downstream direction. The water table remains relatively constant in these deposits and does not fluctuate as rapidly as does the shallow water table in consolidated deposits.
Storage coefficients (ratio of the volume of water a rock will yield by gravity to its own volume) of the alluvial deposits are much higher than those in the consolidated rocks, and where sufficient saturated thickness occurs, dependable water supplies can be developed. Yields of 15 to 20 gpm from these deposits are not uncommon. Yields of 90 to 100 gpm can be obtained in parts of the aquifer.
The water obtained from the Wisconsinan and Recent alluvial aquifer is usually very hard calcium bicarbonate water with a high iron content. It would require treatment for some uses.
Other stream valleys--Deposits of alluvium in other stream valleys in Allen County are thin and yield only small water supplies to wells. The alluvium in many of these tributary streams is so thin and discontinuous that it is not shown on plate 1.
Storage
Surface Water
At the present time (1965), storage of surface water in Allen County is limited to farm ponds and two overflow dams in the channel of the Neosho River. In 1965, Allen County had about 2,459 farm ponds with an approximate storage capacity of 1,850 acre-feet of water (C. E. Crews, written commun., 1966).
Ground Water
Williams (1944) estimated in an earlier study of alluvium in the Neosho River valley near Parsons, Kans., that about 680 million gallons (2,100 acre-feet) of ground water was in storage per square mile of alluvium. This amount was determined by using an average thickness of water-bearing material in the alluvium of 17 feet and a storage coefficient of 20 percent. In Allen County in 1964, the average thickness of saturated alluvial deposits was about 8 feet. Using a storage coefficient of 20 percent and a saturated thickness of 8 feet, about 320 million gallons (980 acre-feet) of water per square mile of alluvium is in storage in the Neosho River valley in Allen County. Wisconsinan and Recent alluvial deposits in the Neosho River valley in Allen County underlie an area of about 54 square miles and contain about 17,300 million gallons (53,000 acre-feet) of water in storage.
If at least 5 percent of the mean annual precipitation (36.93 inches) in the valley recharges the aquifer, then about 35 million gallons (110 acre-feet) per square mile is added annually to the aquifer (Williams, 1944). Much of this, however, is probably transpired by plants or discharged into the Neosho River.
Chemical Character of Ground Water
General Discussion
Water quality-controlling factors--Water falling on the earth's surface absorbs gases from the atmosphere and from the soil. Absorption of these gases enables the water percolating to the aquifer to dissolve minerals from the rocks it contacts. The amount and type of minerals dissolved are determined by such factors as mineralogical composition of the rocks, solubility of the minerals contained, and rate of ground-water movement through the aquifer. These factors control the chemical quality of the water that characterizes an aquifer. Because these factors are variable, the chemical quality of ground water generally differs from place to place.
Properties of water determined by chemical analysis--The kind and amount of dissolved minerals in ground water that determines corrosiveness, encrusting tendency, palatability, and other objectionable or desirable properties can be determined from the results of quantitative chemical analyses.
The analyses of 57 samples of water from wells and streams in Allen County are given in table 2. The concentrations of dissolved chemical constituents are expressed in parts per million (ppm). One part per million is 1 unit weight of constituent in 1 million unit weights Of water. Generally, when describing the chemical composition of water and the relations of ions in solution, units of equivalents per million are more convenient to work with than units of parts per million. One equivalent per million is 1 unit chemical combining weight of a constituent in 1 million unit weights of water. Factors for the conversion of parts per million of mineral constituents to equivalents per million are given in table 3.
Table 2--Chemical analyses of water from selected wells and streams. [Dissolved constituents and hardness in parts per million.]
| Well number (1) |
Sample no. (figs 11-13) |
Depth, feet |
Geologic source (2) |
Date of collection |
Temp. (°F) |
Dissolved solids (evporated at 180° C) |
Silica (SiO2) |
Total Iron (Fe) |
Manganese (Mn) |
Calcium (Ca) |
Magnesium (Mg) |
Sodium (Na) |
Potassium (K) |
Bicarbonate (HCO3) |
Sulfate (SO4) |
Chloride (Cl) |
Fluoride (F) |
Nitrate (NO3) (3) |
Hardness as CaCO3 | Specific conductance (micromhos at 25° C) |
pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Non- carbonate |
|||||||||||||||||||||
| 23-18E-31ccc | 1 | 19 | Illinoisan St |
3-5-65 | 47 | 324 | 12 | 2.0 | 0.05 | 91 | 11 | 15 | 0.8 | 307 | 35 | 6.0 | 0.1 | 1.8 | 272 | 20 | 530 | |
| 23-19E-26cad | 2 | Stream | 5-13-64 | 56 | 265 | 16 | 70 | 7.2 | 14 | 5.4 | 251 | 23 | 5.0 | .4 | .9 | 204 | 0 | 430 | ||||
| 23-19E-29bba | 3 | Stream | 5-13-64 | 62 | 261 | 8.0 | 75 | 7.0 | 11 | 3.3 | 232 | 24 | 18 | .3 | .5 | 216 | 26 | 440 | ||||
| 23-20E-33aad | 4 | 190 | Chanute Sh |
3-5-65 | 49 | 1,961 | 12 | .41 | .15 | 176 | 171 | 208 | 12 | 395 | 1,016 | 169 | 2.0 | .2 | 1,142 | 818 | 2,670 | |
| 23-21E-32aba | 5 | 148 | Cherryvale Sh |
3-5-65 | 51 | 3,812 | 7.5 | .24 | .00 | 27 | 19 | 1,440 | 11 | 466 | 37 | 2,030 | 5.5 | 5.3 | 146 | 0 | 7,020 | |
| 24-17E-1bbb1 | 6 | Stream | 5-13-64 | 64 | 257 | 13 | 54 | 9.1 | 22 | 4.4 | 173 | 52 | 16 | .4 | 1.0 | 172 | 30 | 410 | ||||
| 24-17E-12bad | 7 | Stream | 5-13-64 | 66 | 234 | 11 | 54 | 11 | 10 | 4.7 | 166 | 44 | 13 | .4 | 4.4 | 180 | 44 | 380 | ||||
| 24-18E-5ccb | 8 | 30 | Ilinoisan St |
3-5-65 | 55 | 403 | 14 | .00 | .00 | 118 | 11 | 14 | .8 | 342 | 32 | 21 | .1 | 24 | 340 | 60 | 700 | |
| 24-18E-10dcc | 9 | Stream | 5-13-64 | 62 | 260 | 7.5 | 75 | 6.1 | 10 | 4.2 | 229 | 29 | 15 | .4 | .4 | 212 | 24 | 440 | ||||
| 24-18E-17aad | 10 | 17 | Quaternary Sys |
3-5-65 | 54 | 347 | 11 | .00 | .00 | 102 | 6.2 | 17 | .8 | 303 | 36 | 7.0 | .1 | 18 | 280 | 32 | 580 | |
| 24-18E-21bdb | 11 | Stream | 5-13-64 | 67 | 230 | 10 | 53 | 6.8 | 14 | 4.7 | 168 | 43 | 12 | .4 | 3.1 | 160 | 22 | 370 | ||||
| 24-18E-28ccc | 12 | 19 | Wisconsinan St |
7-29-64 | 507 | 15 | 1.8 | .28 | 142 | 21 | 454 | 77 | 15 | 1 | .0 | 441 | 69 | 760 | ||||
| 24-18E-32ddd | 13 | 44 | Iola Ls | 3-26-65 | 1,982 | 18 | 2.2 | .00 | 218 | 77 | 322 | 2.3 | 566 | 692 | 161 | .4 | 212 | 860 | 396 | 2,500 | ||
| 24-18E-36aaa | 14 | Stream | 5-13-64 | 62 | 197 | 15 | 39 | 7.9 | 13 | 5.4 | 149 | 31 | 7.0 | .5 | 4.4 | 130 | 8 | 310 | ||||
| 24-19E-9ccc | 220 | Dennis Ls |
7-20-64 | 60 | 3,900 | |||||||||||||||||
| 24-19E-14daa | 15 | Stream | 5-13-64 | 63 | 262 | 9.0 | 62 | 13 | 10 | 9.0 | 232 | 29 | 10 | .5 | 5.3 | 208 | 18 | 430 | ||||
| 24-20E-1dda | 16 | 110 | Cherryvale Sh |
3-5-65 | 48 | 1,254 | 9.8 | .00 | .04 | 93 | 38 | 314 | 3.2 | 364 | 220 | 391 | 3.2 | 2.4 | 388 | 90 | 2,200 | |
| 24-20E-22ddc | 17 | 55 | Iola Ls | 3-5-65 | 46 | 793 | 8.2 | 5.7 | .12' | 120 | 34 | 109 | 1.3 | 415 | 259 | 43 | .2 | 14 | 439 | 99 | 1,200 | |
| 24-20E-31ccd | 18 | 200 | Dennis Ls | 3-5-65 | 47 | 378 | 7.8 | 3.5 | .02 | 50 | 12 | 78 | 116 | 53 | .9 | 44 | 174 | 110 | 610 | |||
| 24-20E-34* | Mississippian Sys |
7,400 (4) |
8.0 | .00 | 138 | 59 | 2,688 (5) |
588 | 4 | 4,218 | ||||||||||||
| 24-20E-36* | 160 | Swope Ls | 2,410 (4) |
8.0 | .00 | .00 | 48 | 37 | 846 (5) |
564 | 78 | 1,113 | ||||||||||
| 24-21E-15dcd | 19. | 56 | Cherryvale Sh |
3-5-65 | 59 | 379 | 12 | .19 | .00 | 86 | 27 | 18 | .5 | 383 | 21 | 9.0 | 2.2 | 15 | 326 | 12 | 650 | |
| 24-21E-34* | 1,035 | Arbuckle Gp |
4,090 (4) |
22 | 17 | 76 | 34 | 1,480 (5) |
515 | 21 | 2,199 | |||||||||||
| 25-17E-35ddd | 20 | 235 | Chanute Sh |
3-26-65 | 4,280 | 8.2 | 1.8 | .00 | 70 | 33 | 1,560 | 12 | 630 | 107 | 2,175 | 4.4 | .3 | 310 | 0 | 6,700 | ||
| 25-18E-8aaa | 21 | 90 | Iola Ls | 3-26-65 | 50 | 978 | 20 | .03 | .00 | 178 | 26 | 109 | 1.3 | 129 | 314 | 38 | .2 | 80 | 551 | 199 | 1,230 | |
| 25-18E-9acc1 | 22 | Stream | 5-13-64 | 69 | 228 | 11 | 54 | 9.6 | 10 | 4.7 | 163 | 44 | 12 | .4 | 2.3 | 174 | 40 | 380 | ||||
| 25-18E-14ccb | 23 | 220 | Dennis Ls |
3-26-65 | 1,139 | 5.7 | .01 | .00 | 248 | 26 | 51 | 3.8 | 264 | 186 | 76 | .2 | 412 | 726 | 510 | 1,470 | ||
| 25-19E-6ccb1 | 24 | Stream | 5-13-64 | 68 | 214 | 11 | 56 | 5.0 | 9.2 | 4.4 | 159 | 39 | 9.0 | .4 | 1.6 | 160 | 30 | 340 | ||||
| 25-19E-6ccb2 | 25 | 65 | Iola Ls | 3-5-65 | 53 | 1,152 | 16 | .01 | .00 | 136 | 42 | 192 | 1.8 | 437 | 317 | 112 | .2 | 120 | 512 | 154 | 1,740 | |
| 25-19E-7cdc | 195 | Iola Ls, Chanute Sh |
3-5-65 | 52 | 2,848 | 7.2 | .36 | .00 | 18 | 15 | 1,092 | 8.0 | 805 | 86 | 1,170 | 11 | .8 | 106 | 0 | 5,080 | ||
| 25-19E-14aab | 26 | 150 | Cherryvale Sh |
3-26-65 | 44 | 1,377 | 6.0 | 2.0 | .00 | 13 | 1.8 | 544 | 3.8 | 605 | 16 | 435 | 16 | .7 | 40 | 0 | 2,210 | |
| 25-19E-24cdc | 27 | 225 | Cherryvale Sh |
6-29-65 | 272 | 16 | 5.7 | .70 | 51 | 10 | 20 | 20 | 239 | 11 | 22 | .6 | 3.6 | 168 | 0 | 450 | 7.3 | |
| 25-20E-10ccb | 120 | Chanute Sh |
3- 5-65 | 54 | 635 | 6.8 | .00 | .00 | 10 | 5.6 | 233 | 2.0 | 551 | 39 | 33 | 3.5 | 31 | 48 | 0 | 1,060 | ||
| 25-20E-25baa | 28 | 38 | Cherryvale Sh |
3-26-65 | 45 | 1,914 | 6.8 | .13 | .46 | 266 | 102 | 174 | 21 | 285 | 824 | 149 | .6 | 230 | 1,082 | 848 | 2,390 | |
| 25-20E-27bbc | 165 | Cherryvale Sh |
3-26-65 | 50 | 1,081 | 7.8 | .00 | .00 | 11 | 6.9 | 398 | 3.3 | 473 | 136 | 276 | 9.0 | .2 | 56 | 0 | 1,760 | ||
| 25-20E-30ddc | 29 | 200 | Cherryvale Sh, Dennis Ls |
3-26-65 | 50 | 4,072 | 7.8 | .20 | .00 | 48 | 28 | 1,515 | 11 | 517 | 53 | 2,145 | 9.0 | .2 | 235 | 0 | 6,700- | |
| 25-21E-21* | 100 | Dennis Ls |
8.0 | 3.0 | 17 | 18 | 210 (5) |
342 | 22 | 177 | ||||||||||||
| 25-21E-32aab | 30 | 70 | Dennis Ls |
6-30-65 | 62 | 434 | 7.0 | 2.7 | .00 | 77 | 45 | 22 | 1.0 | 373 | 73 | 22 | 2.2 | 1.6 | 377 | 71 | 730 | 7.8 |
| 26-18E-2dab | 31 | Stream | 5-13-64 | 67 | 224 | 10 | 61 | 4.4 | 9.5 | 3.8 | 188 | 33 | 7.0 | .3 | 2.4 | 170 | 16 | 370 | ||||
| 26-18E-12* | Arbuckle Gp |
8.0 | 9. | 302 | 156 | 11,998 (5) |
616 | 3 | 19,148 | |||||||||||||
| 26-18E-13cbc | 32 | 120 | Iola Ls | 6-28-65 | 65 | 911 | 9.8 | .87 | .27 | 203 | 21 | 38 | 52 | 368 | 88 | 132 | .1 | 186 | 593 | 291 | 1,440 | 7.5 |
| 26-18E-20* | 1,054 | Mississippian Sys |
20 | 8. | 107 | 70 | 4,853 (5) |
578 | 8 | 7,229 | ||||||||||||
| 26-18E-22* | 1,385 | Arbuckle Gp |
18 | 118 | 55 | 2,746 (5) |
610 | 6 | 4,245 | |||||||||||||
| 26-18E-22aa* | Arbuckle Gp |
14 | 46 | 48 | 2,517 (5) |
714 | 3 | 3,688 | ||||||||||||||
| 26-18E-29aac1 | 33 | Stream | 5-13-64 | 68 | 235 | 11 | 54 | 11 | 10 | 4.7 | 168 | 43 | 14 | .4 | 3.6 | 180 | 42 | 390 | ||||
| 26-18E-32ccc | 34 | 22 | Chanute Sh |
3-26-65 | 50 | 2,386 | 13 | 1.1 | .00 | 576 | 42 | 106 | 3.0 | 351 | 1,376 | 88 | .6 | 8.0 | 1,610 | 1,322 | 2,500 | |
| 26-18E-34* | 1,000 | Arbuckle Gp |
10 | 3. | 452 | 369 | 8,745 (5) |
631 | 5 | 15,071 | ||||||||||||
| 26-19E-7aaa | 35 | 200 | Dennis Ls, Cherryvale Sh |
6-28-65 | 68 | 988 | 6.8 | 53 | .48 | 138 | 30 | 121 | 31 | 300 | 156 | 105 | .3 | 252 | 468 | 222 | 1,480 | 7.7 |
| 26-19E-27bbc | 36 | 130 | Cherryvale Sh |
3-26-65 | 50 | 824 | 9.8 | .20 | .00 | 46 | 15 | 238 | 4.0 | 422 | 207 | 91 | 3.2 | 2.4 | 176 | 0 | 1,220 | |
| 26-19E-36cdd | 37 | Stream | 5-13-64 | 65 | 210 | 9.5 | 56 | 6.9 | 7.5 | 3.5 | 159 | 37 | 7.0 | .4 | 3.2 | 168 | 38 | 340 | ||||
| 26-20E-1bab | 38 | 120 | Dennis Ls |
6-29-65 | 72 | 950 | 14 | .21 | .11 | 154 | 59 | 81 | 19 | 454 | 218 | 117 | 2.0 | 62 | 626 | 254 | 1,520 | 7.8 |
| 26-20E-13bba | 39 | 160 | Dennis Ls |
6-30-65 | 64 | 2,896 | 13 | 1.4 | .0 | 352 | 105 | 395 | 2.4 | 298 | 1,306 | 213 | .5 | 363 | 1,310 | 1,066 | 3,690 | 7.4 |
| 26-20E-17aaa | 40 | 160 | Cherryvale Sh |
6-30-65 | 62 | 697 | 14 | 3.1 | .12 | 104 | 29 | 103 | 1.6 | 432 | 156 | 53 | .3 | 24 | 378 | 24 | 1,100 | 7.6 |
| 26-20E-15* | Mississippian Sys |
3,440 (4) |
28 | 8. | 88 | 48 | 1,199 (5) |
581 | 35 | 1,808 | ||||||||||||
| 26-21E-15aba | 41 | 91 | Dennis Ls |
6-30-65 | 64 | 1,318 | 9.8 | .96 | .0 | 189 | 39 | 172 | 1.4 | 354 | 304 | 105 | 1.0 | 323 | 632 | 342 | 1,880 | 8.0 |
| 26-21E-28aaa | 42 | 105 | Dennis Ls |
6-30-65 | 63 | 514 | 13 | .07 | 0. | 53 | 51 | 64 | 2.0 | 420 | 35 | 43 | 2.4 | 44 | 342 | 0 | 860 | 7.8 |
| 26-21E-29* | 1,640 | Arbuckle Gp |
5,860 (4) |
12 | 192 | 83 | 1,971 (5) |
383 | 41 | 3,369 | ||||||||||||
| (1) Analyses with asterisk after well number are by Oilfield Research Laboratory, Chanute, Kans. All other analyses are by Kansas State Department of Health. (2) Gp, Group; Ls, Limestone; Sh, Sliale; St, Stage; Sys, System. (3) In areas where the nitrate content of water is known to exceed 45 ppm, the public should be warned of the potential dangers of using the water for infant feeding (U.S. Public Health Service, 1962, p. 7). (4) Dissolved solids are calculated. (5) Sodium (Na) and potassium (K) are calculated and reported as sodium. |
||||||||||||||||||||||
Table 3--Factors for converting parts per million to equivalents per million.
| Mineral constituents | Chemical symbol | Factor |
|---|---|---|
| Cations | ||
| Calcium | Ca++ | 0.04990 |
| Magnesium | Mg++ | 0.08220 |
| Sodium | Na+ | 0.04350 |
| Potassium | K+ | 0.02558 |
| Anions | ||
| Carbonate | CO3-- | 0.03333 |
| Bicarbonate | HCO3- | 0.01639 |
| Sulfate | SO4-- | 0.02082 |
| Chloride | Cl- | 0.02820 |
| Fluoride | F- | 0.05263 |
| Nitrate | NO3- | 0.01613 |
Methods used in this report--A Piper 1944) diagram is a graphical method used to compare and interpret water-quality data. Figure 11 is a modified Piper diagram of percent equivalents per million of cations and anions for selected water samples in Allen County. The scale of the cations, calcium plus magnesium, ranges from 0 on the left side of the diagram to 100 percent on the right, whereas sodium plus potassium ranges from 0 on the right to 100 percent on the left. The scale of the anions, chloride plus sulfate plus nitrate, ranges from 0 at the bottom to 100 percent at the top, whereas bicarbonate plus carbonate ranges from 0 at the top to 100 percent at the bottom.
Figure 11--Modified Piper diagram of percent equivalents per million of cations and anions for selected water samples. Small numbers by symbols are sample-identification numbers from table 2. Large circled numbers indicate areas of a specific chemical type of water.
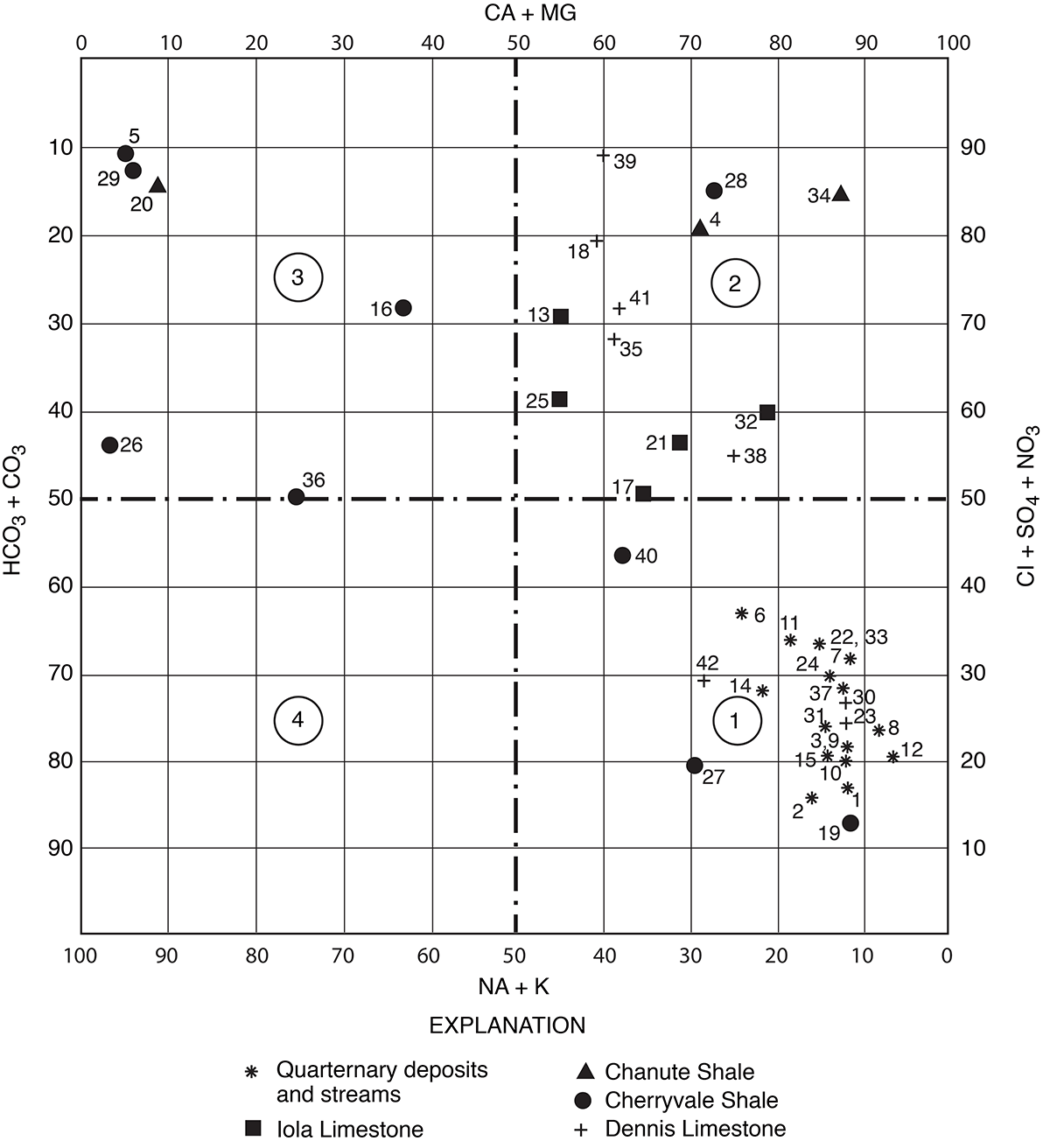
Generally, waters are classified as to chemical type based on the abundance of specific cations and anions in solution. A calcium bicarbonate water is one in which the calcium exceeds 50 percent of the cations and bicarbonate exceeds 50 percent of the anions, based on equivalents per million. For water in which no cation or anion is predominant, the classification is based on the order of abundance of the specific ions, such as calcium magnesium bicarbonate. The percentage equivalents per million of a constituent (cation or anion) is the ratio of the equivalents per million of the constituent to the total equivalents per million of cations or anions.
Large circled numbers on figure 11 indicate areas of a specific chemical type of water. For example, the principal cations and anions for a water that plots in quadrant 1 are calcium plus magnesium and bicarbonate plus carbonate, respectively. The percentage of calcium plus magnesium exceeds that of sodium plus potassium in most of the samples. Bicarbonate and carbonate comprise more than 50 percent of the anions in water from streams and Quaternary deposits. Chloride, sulfate, and nitrate are the principal anions in water from the bedrock aquifers (fig. 11).
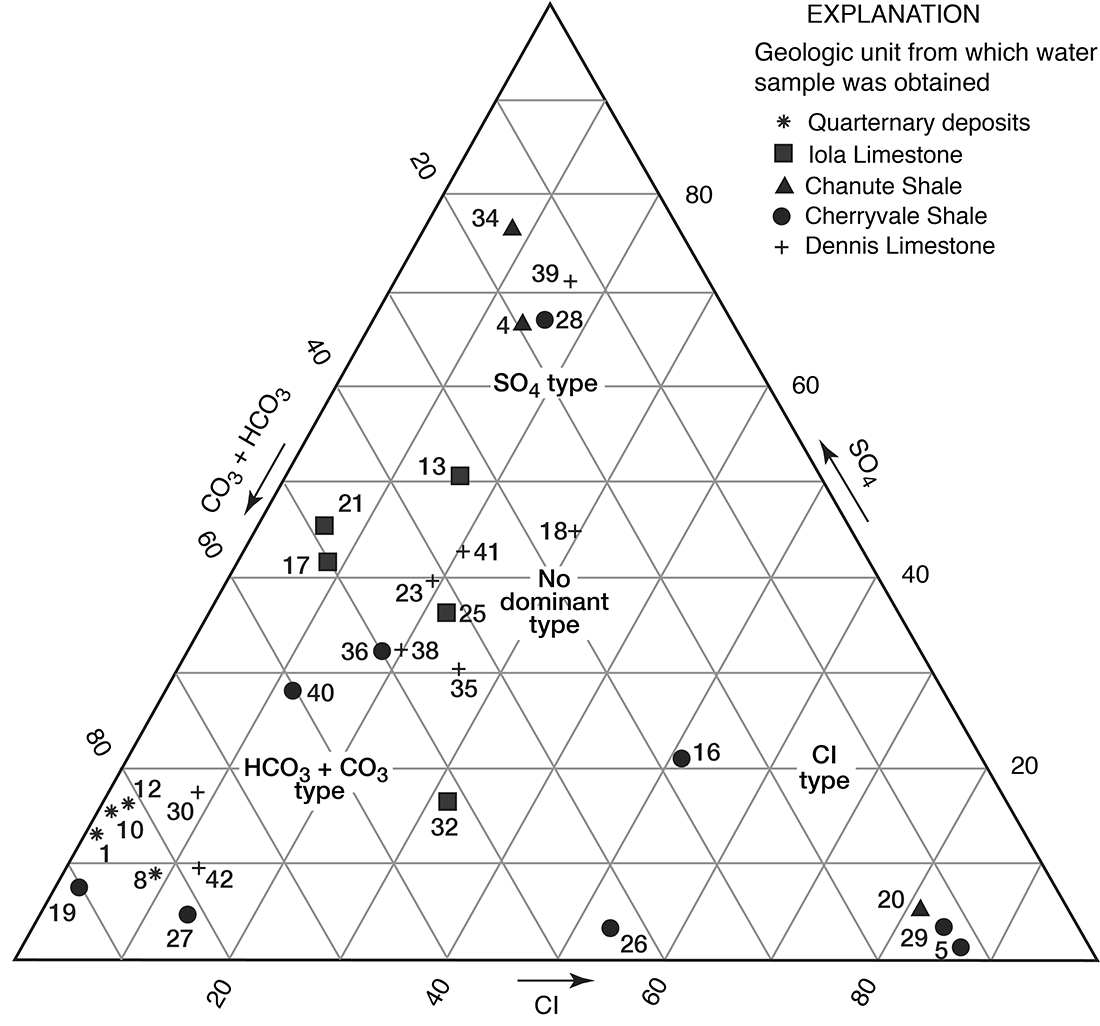
Figures 12 and 13 are trilinear diagrams of percent equivalents per million of cations and anions for selected water samples in Allen County. Most of the data for water samples from limestone aquifers plot in the "no dominant type" area on the cation and anion plots. The data for water samples from shale aquifers show a varied pattern when plotted, but many fall into the sodium, chloride, and sulfate areas on figures 12 and 13. Magnesium is never the predominant cation, although in samples 4 and 42 it is the principal cation.
Figure 12--Trilinear diagram of percent equivalents per million of cations for selected water samples. Numbers by symbols are sample-identification numbers from table 2.
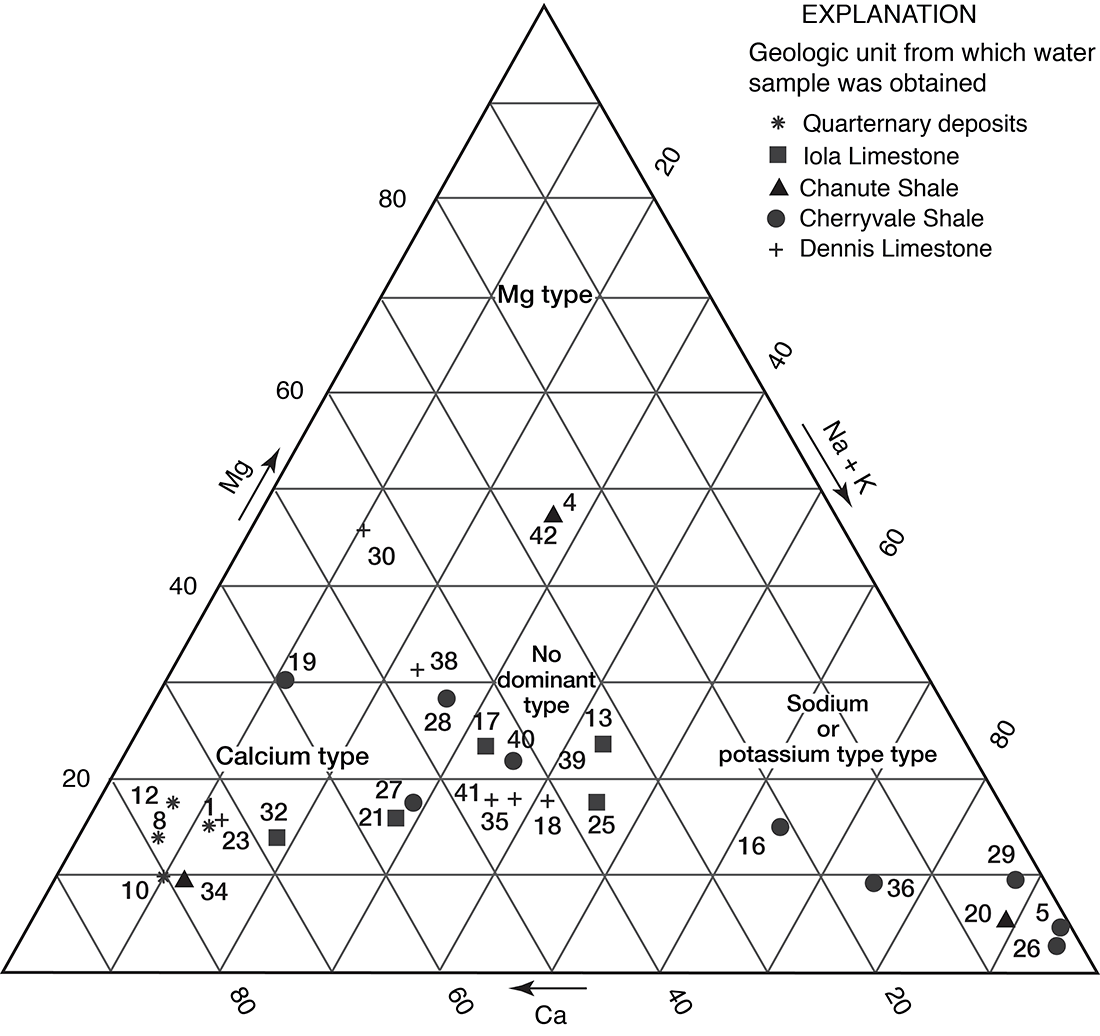
Figure 13--Trilinear diagram of percent equivalents per million of anions for selected water samples. Numbers by symbols are sample-identification numbers from table 2.
Chemical Quality of Water in Relation to Source
Formations of Pre-Kansas City Age
Potable ground water is generally not found in Allen County below the base of the Kansas City Group, and in many places the boundary between fresh and saline water is much higher in the stratigraphic section.
Samples from wells drilled into Lower Pennsylvanian, Mississippian, and Ordovician rocks have one common characteristic: all are high in sodium and chloride content. Table 2 includes water analyses from representative wells in Mississippian limestones and Arbuckle rocks.
The high chloride content of waters in rocks below the base of the Kansas City Group is probably due either to saline connate water or to concentration of chloride by some physical or chemical mechanism.
Very little is known about the artesian pressures of ground water in formations of pre-Kansas City age in Allen County. However, several reports indicate that water under artesian pressure from Mississippian and Ordovician rocks rises to within 20 feet of the land surface. One well in Bourbon County with a static water level 188 feet below land surface encountered the Jefferson City Dolomite at 1,461 feet.
Consolidated Rocks
Certain problems arise in interpreting the chemical geohydrology of the consolidated aquifers in Allen County. The depth at which water is entering a well is usually difficult to determine; all the rocks penetrated by a well could conceivably yield some water to that well. Owing to vast differences in permeabilities of the units penetrated, some geologic units might yield water at such a slow rate that the water is undetected at the time the well is drilled. The chemical quality of water pumped from a well could vary seasonally because of a variation in the percentage of water from each aquifer in a multi-aquifer well. Also, water from different horizons within the same aquifer could have different chemical characteristics.
Little is known concerning hydraulic heads in the consolidated rocks in Allen County. Head differentials in these rocks control to a great extent the flow pattern and, hence, the quality of the water. Certain inferences, however, can be made from the available data, which may explain to some extent certain aspects of the geochemistry of the water in these units.
Certain areas in the county have water of poor quality, an insufficient quantity of water, or both. The northwestern part of the county is underlain by consolidated deposits that are relatively impermeable and yield very little water to wells. Locally, however, wells yielding sufficient quantities of water for domestic use are drilled in this area. The shallow ground water in this area is locally high in chloride content, and the probability of getting an adequate supply of potable water is poor. In the extreme southwestern part of the county, in T. 26 S., R. 17 E., residents in the upland areas have difficulty in obtaining potable water; many of the people use cisterns and surface-water sources. Ground water in this area is characterized by a high chloride concentration. Ground water in an area in the central part of T. 25 S., R. 19 E., the western half of T. 25 S., R. 20 E., the extreme northeastern part of T. 26 S., R. 19 E., and the northwestern part of T. 26 S., R. 20 E., also contains high chloride concentrations at shallow geologic horizons.
Past exploration for and exploitation of gas and oil in the area may have contributed to the high concentrations of chloride in waters found in the rocks above the base of the Kansas City Group in Allen County.
Limestone Aquifers
Most of the limestones in the county yield water in varying amounts. Only a few limestones, however, have a sufficiently wide outcrop band or sufficient permeability to be considered as important bedrock aquifers. The units in Allen County that have not been considered here are limited in their reliability as aquifers by such factors as insufficient thickness, low permeability, and limited outcrop area. The quality of the water from these limestones is poor except in local areas. A fairly good correlation seems to exist between quantity and quality of water in these limestones. Generally, the quality improves with increasing availability of water in the aquifer.
Dennis Limestone--Water from the Dennis Limestone is generally of the calcium bicarbonate sulfate type. However, some of the analyses of samples from wells in Allen County indicate that a high percentage composition of magnesium, sodium, and chloride occurs locally. Total ionic concentration varied widely; dissolved solids concentrations ranged from 378 to 2,896 PPM.
In the outcrop area, the Dennis Limestone contains some dolomite with a Ca/Mg ratio of 1. The ratios of water sampled from the Dennis in this area range from 0.6 to 1.5. As the water moves downdip to the north and to the west, some base exchange apparently occurs and, locally, sodium is exchanged for calcium.
High concentrations of sulfate are characteristic of the water from some wells in the Dennis in Allen County. These wells penetrate thin beds of black fissile shale in the upper part of the Winterset Limestone Member of the Dennis Limestone. Many of the wells also penetrate the Stark Shale Member of the Dennis, which is also black and fissile. Phosphatic nodules [Ca5(PO5)3F] are scattered throughout these shales. The presence of high fluoride and phosphate content, as well as high sulfate content, in the water sampled indicates that the water probably Is derived from the black shales.
In the outcrop area, wells drilled into the Dennis obtain water of excellent quality.
Water from depths greater than 100 feet generally contain lower concentrations of dissolved solids if the water is from the Dennis than if it is from other limestone aquifers, but water from a well drilled into the Dennis in the SW SW SW sec. 9, T. 24 S., R. 19 E., had a chloride content of 3,900 ppm at a depth of 220 feet.
Nitrate in concentrations in excess of 250 ppm was found in four water samples from wells obtaining water from the Dennis in Allen County (table 2). Polluted water from a shallower horizon probably is entering these wells.
Iola Limestone--Figure 11 shows that all the water samples from the Iola plot in area 2 of the Piper diagram, as do most of the waters sampled from limestone aquifers in Allen County. The principal cations are calcium and magnesium; the principal anions are sulfate and chloride. As mentioned previously, the extensive joint system in the Iola Limestone seems to give that unit fairly good horizontal and vertical permeability. This, and the fact that a large area of the county is directly underlain by the Iola Limestone, makes it one of the more important bedrock aquifers in Allen County. The fact that most of these joints are open for water movement indicates that the water is unsaturated with respect to calcium and bicarbonate. Water saturated with respect to calcium and bicarbonate would leave deposits in the open joints and decrease the permeability.
Water from shallow wells in the Iola in the outcrop area locally is relatively high in sulfate content. The exact mechanism for the formation of sulfate water at this horizon is not known. No insoluble residue data are available for the Iola Limestone in Allen County, but in counties to the north finely disseminated pyrite (FeS2) does exist in the Iola (Miller, 1966). If finely disseminated pyrite were oxidized to form a soluble sulfate, the slightly acidic water would tend to keep deposits of calcium carbonate (CaCO3) from forming in the joints. Gypsum in the form of selenite crystals occurs locally in the Muncie Creek Shale Member of the Iola. Solution of the gypsum by water moving through the Iola could increase the sulfate content of the ground water.
Locally, wells drilled into the Iola will yield 5 to 20 gpm of saline water. The magnitude of these well yields could result from the fact that saline water will cause more limestone solution, and hence more permeability, than fresh water (Back and Hanshaw, 1965).
Near the outcrop and along the strike of the Iola Limestone, water from that unit is of good chemical quality. The concentration of dissolved solids in the Iola increases with depth and distance from the area of recharge.
All but one of the analyses in table 2 containing concentrations of nitrate greater than 45 ppm are for water from limestone aquifers. Four of these analyses are for water samples from wells in the Iola Limestone and contain as much as 212 ppm nitrate. Polluted water possibly reaches the water table through the joint system in the limestones, but such high nitrate concentrations as those found in local areas in eastern Kansas are difficult to explain.
Other limestone aquifers--A few wells in Allen County obtain water from the Swope Limestone. The chemical analysis (table 2) of only one water sample from the Swope Limestone is insufficient to determine the quality of water in the aquifer.
Shale and Sandstone Aquifers
Water in shale and sandstone aquifers in Allen County is usually of poorer quality than water from the limestone aquifers at comparable depths. In part, this is due to the much lower permeabilities of the shales and sandstones. Water in these aquifers moves so slowly that chemical processes in the aquifer have time to completely change the chemical character of the water.
Some water in these aquifers may be connate; that is, water trapped in the sediments during deposition. Because of extremely low permeabilities of the aquifer, this water moves very slowly out of the area. As a result, the shales act as large reservoirs of saline water from which chloride in high concentrations may be slowly diffused to adjoining aquifers.
Bredehoeft and others (1963) in a study of the Illinois basin, an area similar to the Cherokee basin, suggested that a process called clay-membrane concentration was responsible for large amounts of dissolved ions in water from some formations. This process is based on the premise that certain types of clay differentially restrict the passage of ions. The clay particles are negatively charged and repel the anions but are permeable to the cations. Water molecules that do not ionize to the same extent as salt molecules will respond to the differences in hydrostatic head and move upward through the shales if lower hydrostatic heads exist in the overlying beds. If the efficiency of the clay membrane is high, the trapped anions will attract cations and prevent their passage, and over a long period of time large concentrations of these ions will be trapped.
Lane and Bonner Springs and Vilas Shales--Very little water is available from these two units in Allen County. Locally, saturated sandy zones in the shales have enough permeability to yield a few hundred gallons of water per day to wells. The chemical quality of water available from these shales is highly varied.
The Lane and Bonner Springs Shales are essentially an aquiclude, except for a persistent sandy zone near the middle of the unit that yields water to several wells in the north-central part of the county. Several wells in this sandy zone are reported to yield water of fairly good quality.
The Vilas is the better aquifer of the two. Wells in the Vilas obtain small quantities of fairly good-quality water from shallow, large-diameter dug wells, but the shallow wells may become dry during prolonged dry periods.
Cherryvale and Chanute Shales--The Cherryvale and Chanute Shales commonly yield water of the sodium sulfate chloride type. As is characteristic of shale aquifers in Allen County, water from the Cherryvale and Chanute has no definite water-quality pattern on the Piper diagram (fig. 11) or on the trilinear diagrams (figs. 12, 13). Owing to differences in permeability in these shales, very slow mixing of water in a lateral direction within the individual formations is possibly occurring.
Waters from the sandstones in the Cherryvale and Chanute sequence have a rather characteristic chemical quality. They are generally hard and high in concentration of chloride and fluoride; the maximum concentrations reported are 2,175 ppm and 16 ppm, respectively. Shallow wells in the outcrop area of the Cherryvale and Chanute Shales usually obtain water of fairly good quality that becomes progressively poorer downdip.
Unconsolidated Deposits
Deposits in Terrace Position
The chemical quality of water from unconsolidated terrace deposits is highly unpredictable owing to wide ranges in the permeability, topographic position, and varied nature of the rocks directly underlying the deposits. Water from the terrace deposits generally is of good quality, and is similar to that of the alluvial deposits. The water is of the calcium bicarbonate type.
Alluvium in the Neosho Valley
The chemical quality of water in the alluvium in the Neosho River valley is uniformly good, except for local areas where pollution may exist. The data from water samples from wells in these deposits plot in area 1 of figure 11, which indicates a large percentage of calcium plus magnesium relative to sodium plus potassium. The chemical quality of these samples is characteristic of water from these deposits in other counties through which the Neosho River flows. The water from these deposits generally has a hardness of 250 to 450 ppm and may have a high iron content.
Any pollution that becomes apparent in water from wells in the alluvium is probably from a local source. Because of the high permeability of the alluvium and the large quantity of water moving through the aquifer, dilution of any polluting waters may occur a short distance from the point of pollution. Locally in the valley, well water with a high nitrate content has been reported; the aquifer supplying most of these wells is probably being polluted from such sources as sewage effluent and crop fertilizer.
Proper well covering should be provided for wells to prevent surface pollution. The water should be analyzed frequently for nitrate content and for the presence of bacteria. This service can be provided through the County Health Officer at little or no expense to the well owner.
The quality of water from the alluvium in the Neosho Valley is quite similar to the quality of water from the Neosho River, which is expected inasmuch as a good hydraulic connection between the river and the alluvial deposits exists along the entire valley.
In the areas of the county where the alluvium is underlain by the Iola Limestone, water slightly higher in sulfate content has been reported from wells in the alluvium. Water from the Iola Limestone in the west-central part of the county generally has a high sulfate content. Some mixing of waters probably occurs due to an extensive and well-developed joint system where alluvium directly overlies the Iola.
Chemical Quality of Water in Relation to Use
Water-quality criteria differ according to use. Water for domestic purposes should be clear; colorless; free from objectionable odor, taste, and disease-causing micro-organisms; and of reasonable temperature. The significance of certain chemical constituents in ground water and the maximum concentrations recommended for drinking water are given in table 4.
Table 4--Quality of water in relation to use.
| Constituent | Principal characteristics | Acceptable maximum concentration, in parts per million1 |
Range in concentration in ground water in Allen County, in parts per million2 |
|---|---|---|---|
| Dissolved solids | Water high in dissolved -solids content may have a disagreeable taste or have a laxative effect. When water is evaporated, the residue consists mainly of the minerals listed in table 2. | 500--generally satisfactory 1,000--may have noticeable taste or be unsuitable in some other respect |
272-7,400 |
| Hardness3 | Hardness is caused by calcium and magnesium. Forms scale in vessels used in heating or evaporative processes. Hardness is commonly noticed by its effect when soap is used with the water. Carbonate hardness can be removed by boiling; noncarbonate hardness cannot. | 40-1,610 | |
| Total iron (Fe) | Iron stains cooking utensils, plumbing fixtures, and laundry. Water may have disagreeable taste. | 0.3 | 0-53 |
| Fluoride (F) | Fluoride concentrations of about 1 ppm in drinking water used by children during the period of calcification of teeth prevents or lessens the incidence of tooth decay; 1.5 ppm may cause mottling of the tooth enamel (Dean, 1936). Bone changes may occur with concentrations of 8-20 ppm. | 1.5 | 0.1-16 |
| Nitrate (NO3) | Nitrate concentration of 90 ppm may cause cyanosis in infants (Metzler and Stoltenberg, 1950). Comly (1945) states that concentrations of 45 ppm may be harmful to infants. Adverse effects from drinking high-nitrate water are also possible in older children and adults. | 45 | 0-412 (8 samples>90 ppm, 10 samples>45 ppm) |
| Sulfate (SO4) | Derived from solution of gypsum and oxidation of iron sulfides (pyrite, etc.). Concentrations of magnesium sulfate (Epsom salt) and sodium sulfate (Glauber's salt) may have a laxative effect on persons not accustomed to drinking such water. | 250 | 3-1,376 |
| Chloride (Cl) | Chloride in ground water may be derived from connate marine water in sediments, from sewage, or from solution of minerals containing chloride. | 250 | 6.0-19,148 |
| (1) Concentrations as recommended by the U.S. Public Health Service (1962). (2) Based an analyses of 44 ground-water samples (table 2). (3) The hardness of water is classified as follows: 60 ppm or less, soft; 61-120 ppm, moderately hard; 121-180 ppm, hard; and 181 ppm or more, very hard. |
|||
Rigid criteria are not available for evaluating the usefulness of a water as a supply for watering stock. However, most animals seem to be able to use water considerably poorer in quality than would be considered satisfactory for human beings (Hem, 1959).
Use of ground water for agricultural or industrial purposes is very limited in Allen County. However, water-quality requirements for these purposes must be considered. Guidelines for relating the chemical quality to its suitability for irrigation have been proposed (U.S. Salinity Laboratory Staff, 1954). The two main criteria for determining the suitability of water for irrigation are the dissolved-solids content and the sodium concentration relative to the calcium and magnesium concentrations.
Water-quality requirements for industrial use vary depending upon the specific use to be made of the water. Generally, water of a low dissolved-solids content and of a fairly uniform quality will meet the requirements of most industries.
Sanitary Consideration
Well water may have an objectionable taste due to dissolved mineral matter, but it may be free from harmful bacteria and, consequently, safe for drinking. Other well water, good tasting and seemingly pure, may contain harmful bacteria. Pollution may be indicated by excessive amounts of certain dissolved minerals, such as chloride or nitrate.
Recommendations may be obtained from the State Department of Health concerning sanitary construction, locations, and pump installations for different types of wells.
Utilization of Ground Water
Past and Present Use
In Allen County, ground water is used chiefly for domestic and stock purposes. At the present time (1965), no municipal systems use water from ground-water sources. Almost all industry in Allen County uses water from municipal supplies, with the exception of the oil industry, which uses ground water for repressuring in the recovery of oil.
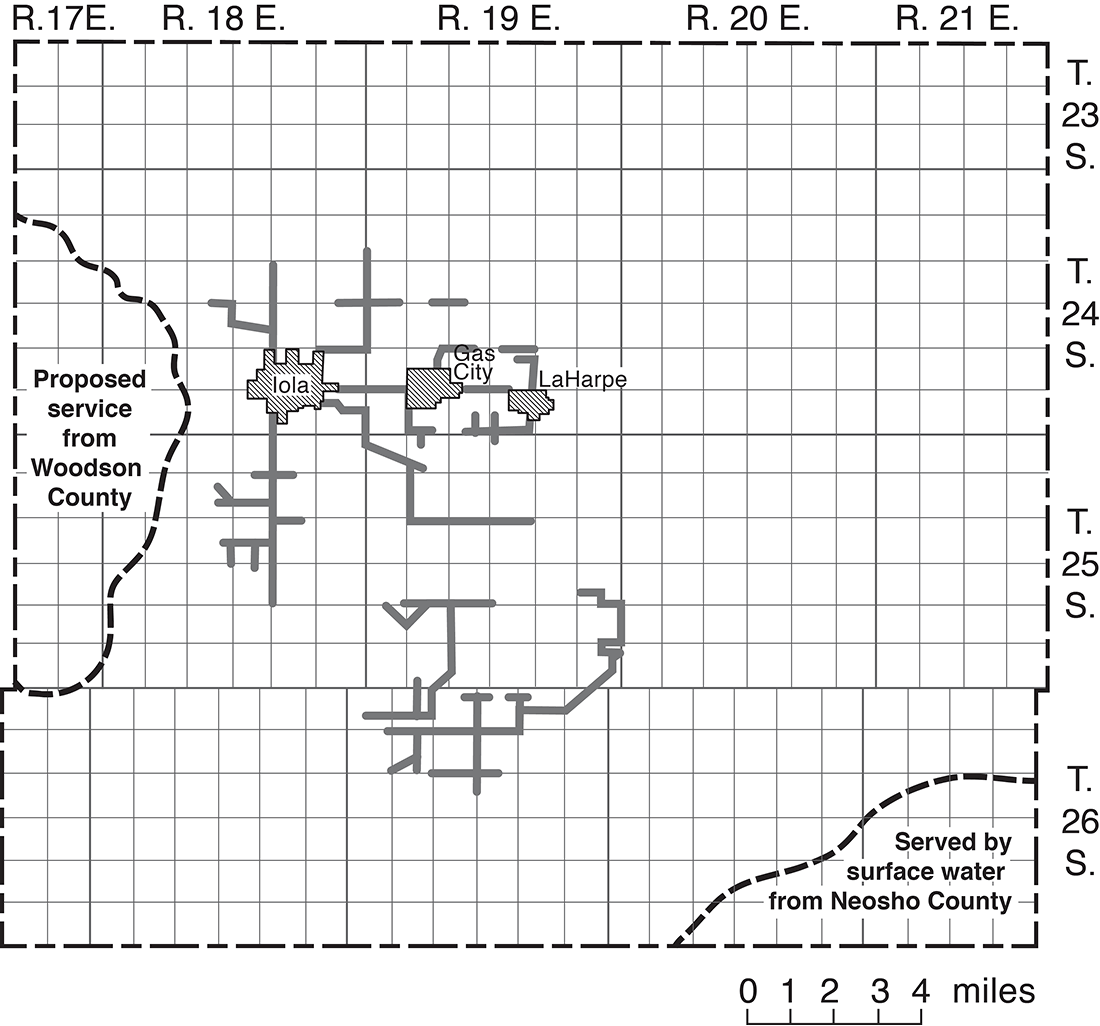
Nearly all domestic and stock water supplies in rural areas are obtained from privately owned wells. In upland areas, ground-water supplies are obtained from dug or drilled wells. Ground water is difficult to obtain in some of the upland areas, and cisterns are used as a source of domestic water on many farms. In valley areas, most supplies are obtained from driven, drilled, or dug wells. Ponds that provide domestic and stock water supplies have been constructed in many places in the county. In June 1965, 147 families were being served by rural water districts that purchased water from the city of Iola, which obtains its water from the Neosho River (Mrs. Elba Bowman, oral commun., 1965). The towns of Gas City and LaHarpe are also served by municipal supplies purchased from Iola (fig. 14).
Figure 14--Areas served by rural water districts (1965).
Potential Use
The largest ground-water potential in the county lies in the Neosho River valley. With proper well location, construction, spacing, and pumping rates, 50 to 100 gpm of water could be pumped from wells in the valley deposits for domestic, stock, irrigation, industrial, or municipal supplies.
The potential of upland limestone, shale, and sandstone aquifers is not as good as that of the unconsolidated aquifers. Large quantities of potable ground water are not available from the consolidated aquifers, and in the foreseeable future these aquifers probably will not be important to the economic development of the county.
Prev Page--Mineral resources || Next Page--Well Records
Kansas Geological Survey, Geology
Placed on web April 14, 2009; originally published December 1969.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/General/Geology/Allen/05_gw.html